
Dong-A ST guarantees consistent product stability by strictly adhering to GMP standards at every stage— from raw material sourcing to production and final shipment.
Before shipment, we conduct comprehensive inspections to detect and address any potential issues, ensuring the highest level of product safety for our customers.


Quality management control
Product complaint and recall
management
Incident and CAPA management
Training and education management
Quality risk management (QRM)
Data integrity management
Manufacturing site, sanitation,
and environment management
Validation management
Manufacturing process
management
Manufacturing equipment
management
Operation of GMP IT systems
(StarLIMS, MES etc.)
Introduction of automated/
digitalized systems
Quality by Design (QbD)
Evaluated raw material suppliers
Evaluated CMOs
Evaluated imported drug suppliers




Motilitone
Opalmon
Sugamet XR

Closerin
Onon
Newrica
Gaster
Cisplan
Perdipine





Growtropin

Growtropin
Leucostim
Eporon
Gonadopin
Dulastin

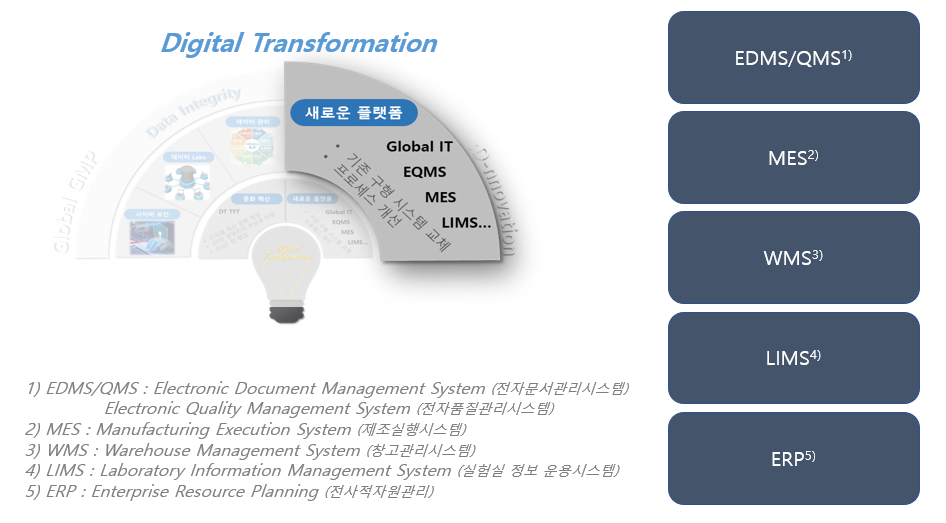
Our Songdo Campus is implemented with an IT system that is compliant with cGMP and Data Integrity regulations.
Suganon
Stillen
Sugamet XR